Effect of processed aloe vera gel on immunogenicity in inactivated quadrivalent influenza vaccine and upper respiratory tract infection in healthy adults: A randomized double-blind placebo-controlled trial |
| |
| Affiliation: | 1. Department of Internal Medicine, Jeonbuk National University Medical School, Jeonju, Jeonbuk 54896, Republic of Korea;2. Research Institute of Clinical Medicine of Jeonbuk National University, Jeonju, Jeonbuk, 54896, Republic of Korea;3. Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, Jeonbuk, 54907, Republic of Korea;4. Clinical Trial Center for Functional Foods, Jeonbuk National University Hospital, Jeonju, Jeonbuk 54907, Republic of Korea;5. Department of Pharmacy, Korea University College of Pharmacy, Sejong 30019, Republic of Korea;6. Department of Infectious Diseases, St. Jude Children''s Research Hospital, Memphis, TN 38105, USA |
| |
| Abstract: | 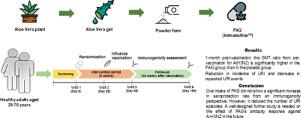
BackgroundAloe vera is a functional food with various pharmacological functions, including an immune-modulating effect. Until now, A. vera has never been studied as an adjuvant in influenza vaccine, and its effects on upper respiratory tract infection (URI) are unknown.PurposeThe objective of our study was to investigate the effect of processed A. vera gel (PAG) on immunogenicity of quadrivalent inactivated influenza vaccine and URI in healthy adults.Study designA randomized, double-blind, placebo-controlled clinical trial was performed.MethodsThis study was conducted in 100 healthy adults at a single center from September 2017 to May 2018. Subjects were randomly divided into a PAG group (n = 50) and a placebo group (n = 50). The enrolled subjects were instructed to ingest the study drug for 8 weeks. The participants received a single dose of quadrivalent inactivated influenza vaccine after taking the study drug for the first 4 weeks of the study. The primary endpoint was seroprotection rate against at least one viral strain at 4 weeks post-vaccination. Other outcomes were seroprotection rate at 24 weeks post-vaccination, seroconversion rate, geometric mean fold increase (GMFI) at 4 and 24 weeks post-vaccination, seroprotection rate ratio and geometric mean titer ratio (GMTR) at 4 weeks post-vaccination between PAG and placebo groups, and incidence, severity, and duration of URI.ResultsThe European Committee for proprietary medicinal products (CPMP) evaluation criteria were met at least one in the PAG and placebo groups for all strains. However, there was no significant difference in the seroprotection rate at 4 weeks post-vaccination against all strains in both PAG and placebo groups. Among secondary endpoints, the GMFI at 4 weeks post-vaccination for the A/H3N2 was significantly higher in the PAG than in placebo group. The GMTR as adjuvant effect was 1.382 (95% CI, 1.014-1.1883). Kaplan–Meier curve analysis showed a reduction in incidence of URI (p = 0.035), and a generalized estimating equation model identified a decrease in repeated URI events (odds ratio 0.57; 95% CI, 0.39-0.83; p = 0.003) in the PAG group.ConclusionsOral intake of PAG did not show a significant increase in seroprotection rate from an immunogenicity perspective. However, it reduced the number of URI episodes. A well-designed further study is needed on the effect of PAG's antibody response against A/H3N2 in the future. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|

