| DNA聚合酶θ:易错的多功能DNA末端修复分子 |
| |
| 作者姓名: | 王瑶 陈国江 冯健男 石艳春 王晶 郑源强 |
| |
| 作者单位: | 1)内蒙古医科大学,内蒙古自治区分子生物学重点实验室,呼和浩特 010058,2)军事科学院军事医学研究院毒物药物研究所,抗毒药物与毒理学国家重点实验室,北京 100850,2)军事科学院军事医学研究院毒物药物研究所,抗毒药物与毒理学国家重点实验室,北京 100850,1)内蒙古医科大学,内蒙古自治区分子生物学重点实验室,呼和浩特 010058,2)军事科学院军事医学研究院毒物药物研究所,抗毒药物与毒理学国家重点实验室,北京 100850,1)内蒙古医科大学,内蒙古自治区分子生物学重点实验室,呼和浩特 010058 |
| |
| 基金项目: | 北京市自然科学基金(7222262) 和国家自然科学基金 (31900676) 资助项目。 |
| |
| 摘 要: | 
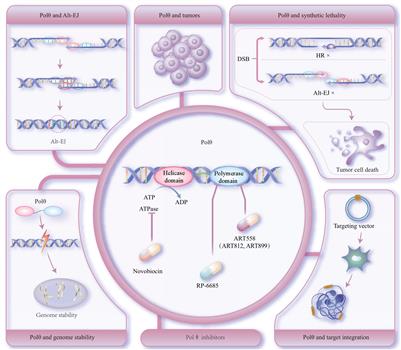
DNA聚合酶θ (DNA polymerase theta,Polθ)是一种广泛存在于动植物中的DNA修复酶。它在选择性末端连接(alternative end-joining,Alt-EJ)途径中发挥着关键作用,常参与DNA双链断裂(DNA double-strand breaks,DSB)损伤修复。在正常生理状态下,Polθ主要调控基因组稳定性。然而,在恶性肿瘤发生时,Polθ表现出异常高表达水平,并参与调控肿瘤细胞的恶性转变过程。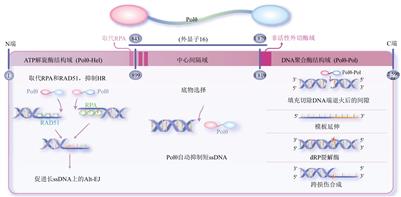
研究表明,抑制Polθ活性可导致同源重组(homologous recombination,HR)缺陷的肿瘤细胞发生合成致死(synthetic lethality,SL)。因此,已经开发出多种针对Polθ的小分子抑制剂,可与其他化疗药物联合使用以抑制恶性肿瘤的发展。此外,敲除或抑制Polθ活性还能增加HR修复效率,从而提高外源基因靶向整合效果。本文综述了Polθ及其介导的Alt-EJ修复机制在生物学功能方面的最新研究进展,为靶向Polθ在肿瘤治疗和基因编辑方面的应用提供理论基础。
|
| 关 键 词: | DNA聚合酶θ DNA双链断裂修复 基因组稳定性 肿瘤抑制 靶向整合 |
| 收稿时间: | 2023-05-22 |
| 修稿时间: | 2024-01-25 |
|
| 点击此处可从《生物化学与生物物理进展》浏览原始摘要信息 |
|
点击此处可从《生物化学与生物物理进展》下载免费的PDF全文 |
|



