Synthesis of 2-(5-bromo-2,3-dimethoxyphenyl)-5-(aminomethyl)-1H-pyrrole analogues and their binding affinities for dopamine D2, D3, and D4 receptors |
| |
| Authors: | Mach Robert H Huang Yunsheng Freeman Rebekah A Wu Li Blair Suwanna Luedtke Robert R |
| |
| Affiliation: | Department of Radiology, PET Center, Wake Forest University School of Medicine, Winston-Salem, NC 27157, USA. rhmach@mir.wustl.edu |
| |
| Abstract: | 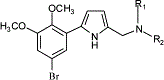
A series of 2-(5-bromo-2,3-dimethoxyphenyl)-5-(aminomethyl)-1H-pyrrole analogues was prepared and their affinity for dopamine D(2), D(3), and D(4) receptors was measured using in vitro binding assays. The results of receptor binding studies indicated that the incorporation of a pyrrole moiety between the phenyl ring and the basic nitrogen resulted in a significant increase in the selectivity for dopamine D(3) receptors. The most selective compound in this series is 2-(5-bromo-2,3-dimethoxyphenyl)-5-(2-(3-pyridal)piperidinyl)methyl-1H-pyrrole (6p), which has a D(3) receptor affinity of 4.3 nM, a 20-fold selectivity for D(3) versus D(2) receptors, and a 300-fold selectivity for D(3) versus D(4) receptors. This compound is predicted to be a useful ligand for studying the functional role of dopamine D(3) receptors in vivo. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect PubMed 等数据库收录! |
|

