2,6-disubstituted pyran-4-one and thiopyran-4-one inhibitors of DNA-Dependent protein kinase (DNA-PK) |
| |
| Authors: | Hollick Jonathan J Golding Bernard T Hardcastle Ian R Martin Niall Richardson Caroline Rigoreau Laurent J M Smith Graeme C M Griffin Roger J |
| |
| Affiliation: | Northern Institute for Cancer Research, School of Natural Sciences-Chemistry, Bedson Building, University of Newcastle, Newcastle Upon Tyne NE1 7RU, UK. |
| |
| Abstract: | 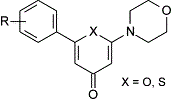
6-aryl-2-morpholin-4-yl-4H-pyran-4-ones and 6-aryl-2-morpholin-4-yl-4H-thiopyran-4-ones were synthesised and evaluated as potential inhibitors of the DNA repair enzyme DNA-dependent protein kinase (DNA-PK). Several compounds in each series exhibited superior activity to the chromenone LY294002, and were of comparable potency to the benzochromenone NU7026 (IC(50)=0.23 microM). Importantly, members of both structural classes were found to be selective inhibitors of DNA-PK over related phosphatidylinositol 3-kinase-related kinase (PIKK) family members. A multiple-parallel synthesis approach, employing Suzuki cross-coupling methodology, was utilised to prepare libraries of thiopyran-4-ones with a range of aromatic groups at the 3'- and 4'-positions on the thiopyran-4-one 6-aryl ring. Screening of the libraries resulted in the identification of 6-aryl-2-morpholin-4-yl-4H-thiopyran-4-ones bearing naphthyl or benzo[b]thienyl substituents at the 4'-position, as potent DNA-PK inhibitors with IC(50) values in the 0.2-0.4 microM range. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect PubMed 等数据库收录! |
|

