Seeking the mechanism responsible for fluoroquinolone photomutagenicity: a pulse radiolysis,steady-state,and laser flash photolysis study |
| |
| Affiliation: | 1. Key Laboratory for Ecological Environment in Coastal Areas (SOA), National Marine Environmental Monitoring Center, Dalian 116023, PR China;2. Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, PR China;1. Department of Environmental Health Sciences, Medical University of Warsaw, Poland;2. Department of Pharmacognosy and Molecular Basis of Phytotherapy, Medical University of Warsaw, Poland;3. Institute of Nuclear Chemistry & Technology, Centre for Radiobiology & Biological Dosimetry, Warsaw, Poland |
| |
| Abstract: | 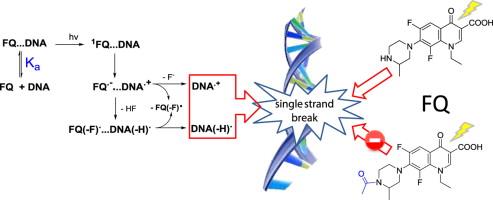
The mechanism responsible for the remarkable photomutagenicity of fluoroquinolone (FQ) antibiotics remains unknown. For this reason, it was considered worthwhile to study in detail the interactions between DNA and a dihalogenated FQ such as lomefloxacin (LFX; one of the most photomutagenic FQs) and its N-acetyl derivative ALFX. Studies of photosensitized DNA damage by (A)LFX, such as formation of DNA single-strand breaks (SSBs), together with pulse radiolysis, laser flash photolysis, and absorption and fluorescence measurements, have shown the important effects of the cationic character of the piperazinyl ring on the affinity of this type of drug for DNA. Hence, the formation of SSBs was detected for LFX, whereas ALFX and ciprofloxacin (a monofluorated FQ) needed a considerably larger dose of light to produce some damage. In this context, it was determined that the association constant (Ka) for the binding of LFX to DNA is ca. 2×103 M−1, whereas in the case of ALFX it is only ca. 0.5×103 M−1. This important difference is attributed to an association between the cationic peripheral ring of LFX and the phosphate moieties of DNA and justifies the DNA SSB results. The analysis of the transient species detected and the photomixtures has allowed us to establish the intermolecular processes involved in the photolysis of FQ in the presence of DNA and 2'-deoxyguanosine (dGuo). Interestingly, although a covalent binding of the dihalogenated FQ to dGuo occurs, the photodegradation of FQ…DNA complexes did not reveal any significant covalent attachment. Another remarkable outcome of this study was that (A)LFX radical anions, intermediates required for the onset of DNA damage, were detected by pulse radiolysis but not by laser flash photolysis. |
| |
| Keywords: | Carcinogenicity DNA Fluoroquinolones Genotoxicity Photolysis Photochemistry Free radicals |
| 本文献已被 ScienceDirect 等数据库收录! |
|

