Pro-oxidative synergic bactericidal effect of NO: kinetics and inhibition by nitroxides |
| |
| Affiliation: | 1. Analytical & Testing Center, Sichuan University, Chengdu, Sichuan 610064, China;2. Analytical & Service Center of Sichuan Province, Chengdu, Sichuan 610023, China;3. College of Chemistry, Sichuan University, Chengdu, Sichuan 610064, China;1. National Institute of Laser Enhanced Sciences (NILES), Cairo University, Egypt;2. Egypt Nanotechnology Center (EGNC), Cairo University, Egypt;3. Egyptian Petroleum Research Institute, Cairo, Egypt;4. Department of Physics, College of Science, Jouf University, Sakaka, Saudi Arabia;5. Chemistry Department, College of Science, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia |
| |
| Abstract: | 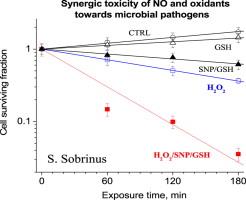
NO plays diverse roles in physiological and pathological processes, occasionally resulting in opposing effects, particularly in cells subjected to oxidative stress. NO mostly protects eukaryotes against oxidative injury, but was demonstrated to kill prokaryotes synergistically with H2O2. This could be a promising therapeutic avenue. However, recent conflicting findings were reported describing dramatic protective activity of NO. The previous studies of NO effects on prokaryotes applied a transient oxidative stress while arbitrarily checking the residual bacterial viability after 30 or 60 min and ignoring the process kinetics. If NO-induced synergy and the oxidative stress are time-dependent, the elucidation of the cell killing kinetics is essential, particularly for survival curves exhibiting a “shoulder” sometimes reflecting sublethal damage as in the linear-quadratic survival models. We studied the kinetics of NO synergic effects on H2O2-induced killing of microbial pathogens. A synergic pro-oxidative activity toward gram-negative and gram-positive cells is demonstrated even at sub-μM/min flux of NO. For certain strains, the synergic effect progressively increased with the duration of cell exposure, and the linear-quadratic survival model best fit the observed survival data. In contrast to the failure of SOD to affect the bactericidal process, nitroxide SOD mimics abrogated the pro-oxidative synergy of NO/H2O2. These cell-permeative antioxidants, which hardly react with diamagnetic species and react neither with NO nor with H2O2, can detoxify redox-active transition metals and catalytically remove intracellular superoxide and nitrogen-derived reactive species such as •NO2 or peroxynitrite. The possible mechanism underlying the bactericidal NO synergy under oxidative stress and the potential therapeutic gain are discussed. |
| |
| Keywords: | Nitric oxide Hydrogen peroxide Synergy Microbial pathogens Oxidative stress Free radicals |
| 本文献已被 ScienceDirect 等数据库收录! |
|

