Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels |
| |
| Authors: | Peterson B Z DeMaria C D Adelman J P Yue D T |
| |
| Affiliation: | Department of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, USA. |
| |
| Abstract: | 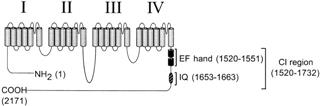
Elevated intracellular Ca2+ triggers inactivation of L-type calcium channels, providing negative Ca2+ feedback in many cells. Ca2+ binding to the main alpha1c channel subunit has been widely proposed to initiate such Ca2+ -dependent inactivation. Here, we find that overexpression of mutant, Ca2+ -insensitive calmodulin (CaM) ablates Ca2+ -dependent inactivation in a "dominant-negative" manner. This result demonstrates that CaM is the actual Ca2+ sensor for inactivation and suggests that CaM is constitutively tethered to the channel complex. Inactivation is likely to occur via Ca2+ -dependent interaction of tethered CaM with an IQ-like motif on the carboxyl tail of alpha1c. CaM also binds to analogous IQ regions of N-, P/Q-, and R-type calcium channels, suggesting that CaM-mediated effects may be widespread in the calcium channel family. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect PubMed 等数据库收录! |
|

