Structural insights into UbiD reversible decarboxylation |
| |
| Affiliation: | Manchester Institute of Biotechnology, Department of Chemistry, University of Manchester, 131 Princess Street, Manchester, M1 7DN, UK |
| |
| Abstract: | 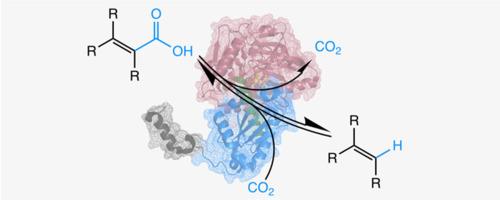
The ubiquitous UbiX-UbiD system is associated with a wide range of microbial (de)carboxylation reactions. Recent X-ray crystallographic studies have contributed to elucidating the enigmatic mechanism underpinning the conversion of α,β-unsaturated acids by this system. The UbiD component utilises a unique cofactor, prenylated flavin (prFMN), generated by the bespoke action of the associated UbiX flavin prenyltransferase. Structure determination of a range of UbiX/UbiD representatives has revealed a generic mode of action for both the flavin-to-prFMN metamorphosis and the (de)carboxylation. In contrast to the conserved UbiX, the UbiD superfamily is associated with a versatile substrate range. The latter is reflected in the considerable variety of UbiD quaternary structure, dynamic behaviour and active site architecture. Directed evolution of UbiD enzymes has taken advantage of this apparent malleability to generate new variants supporting in vivo hydrocarbon production. Other applications include coupling UbiD to carboxylic acid reductase to convert alkenes into α,β-unsaturated aldehydes via enzymatic CO2 fixation. |
| |
| Keywords: | prFMN" },{" #name" :" keyword" ," $" :{" id" :" pc_7xoHpQlTo6" }," $$" :[{" #name" :" text" ," _" :" prenylated flavin mononucleotide DMAP" },{" #name" :" keyword" ," $" :{" id" :" pc_pKzv57fu25" }," $$" :[{" #name" :" text" ," _" :" dimethylallylphosphate DAMPP" },{" #name" :" keyword" ," $" :{" id" :" pc_dZ7LHE1isn" }," $$" :[{" #name" :" text" ," _" :" dimethylallylpyrophosphate |
| 本文献已被 ScienceDirect 等数据库收录! |
|

