
|
|||||
|
|
A series of 5-(5,6)-dihydrouracil substituted 8-hydroxy-[1,6]naphthyridine-7-carboxylic acid 4-fluorobenzylamide inhibitors of HIV-1 integrase and viral replication in cells |
|
| Authors: | Embrey Mark W Wai John S Funk Timothy W Homnick Carl F Perlow Debbie S Young Steven D Vacca Joseph P Hazuda Daria J Felock Peter J Stillmock Kara A Witmer Marc V Moyer Gregory Schleif William A Gabryelski Lori J Jin Lixia Chen I-Wu Ellis Joan D Wong Bradley K Lin Jiunn H Leonard Yvonne M Tsou Nancy N Zhuang Linghang |
| Affiliation: | Department of Medicinal Chemistry, Merck Research Laboratories, West Point, PA 19486, USA. mark_embrey@merck.com |
| Abstract: | 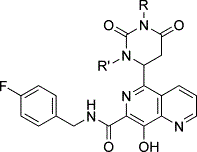 Introduction of a 5,6-dihydrouracil functionality in the 5-position of N-(4-fluorobenzyl)-8-hydroxy-[1,6]naphthyridine-7-carboxamide 1 led to a series of highly active HIV-1 integrase inhibitors. These compounds displayed low nanomolar activity in inhibiting both the strand transfer process of HIV-1 integrase and viral replication in cells. Compound 11 is a 150-fold more potent antiviral agent than 1, with a CIC(95) of 40 nM in the presence of human serum. It displays good pharmacokinetics when dosed in rats and dogs. |
| Keywords: | |
| 本文献已被 ScienceDirect PubMed 等数据库收录! | |