Recombinant expression and characterization of Lucilia cuprina CYP6G3: Activity and binding properties toward multiple pesticides |
| |
| Affiliation: | 1. School of Chemistry and Molecular Biology, University of Queensland, St. Lucia 4072, Australia;2. The Bio21 Institute, The University of Melbourne, Parkville, Victoria, 3010, Australia;1. Nursing Office, North Medical Sector, Linyi People’s Hospital, Linyi 276003, China;2. Department of Rheumatology, Linyi People’s Hospital, Linyi 276003, China;3. Linyi Municipal Health and Family Planning Commission, Linyi 276001, China;4. Department of Gastroenterology, Linyi People’s Hospital, Linyi 276003, China;5. Department of Geratology, Linyi People’s Hospital, Linyi 276003, China;6. The People’s Hospital of Linzi District, Zibo 2255400, China;1. Laboratory of Respiration Physiology, Mossakowski Medical Research Centre Polish Academy of Sciences, A. Pawińskiego 5, 02-106 Warsaw, Poland;2. Department of Neuropeptides, Mossakowski Medical Research Centre Polish Academy of Sciences, A. Pawińskiego 5, 02-106 Warsaw, Poland |
| |
| Abstract: | 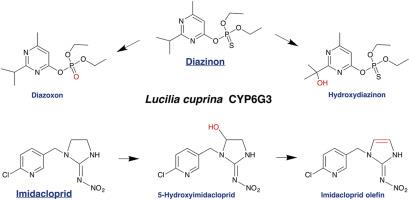
The Australian sheep blowfly, Lucilia cuprina, is a primary cause of sheep flystrike and a major agricultural pest. Cytochrome P450 enzymes have been implicated in the resistance of L. cuprina to several classes of insecticides. In particular, CYP6G3 is a L. cuprina homologue of Drosophila melanogaster CYP6G1, a P450 known to confer multi-pesticide resistance. To investigate the basis of resistance, a bicistronic Escherichia coli expression system was developed to co-express active L. cuprina CYP6G3 and house fly (Musca domestica) P450 reductase. Recombinant CYP6G3 showed activity towards the high-throughput screening substrates, 7-ethoxycoumarin and p-nitroanisole, but not towards p-nitrophenol, coumarin, 7-benzyloxyresorufin, or seven different luciferin derivatives (P450-Glo™ substrates). The addition of house fly cytochrome b5 enhanced the kcat for p-nitroanisole dealkylation approximately two fold (17.8 ± 0.5 vs 9.6 ± 0.2 min−1) with little effect on KM (13 ± 1 vs 10 ± 1 μM). Inhibition studies and difference spectroscopy revealed that the organochlorine compounds, DDT and endosulfan, and the organophosphate pesticides, malathion and chlorfenvinphos, bind to the active site of CYP6G3. All four pesticides showed type I binding spectra with spectral dissociation constants in the micromolar range suggesting that they may be substrates of CYP6G3. While no significant inhibition was seen with the organophosphate, diazinon, or the neonicotinoid, imidacloprid, diazinon showed weak binding in spectral assays, with a Kd value of 23 ± 3 μM CYP6G3 metabolised diazinon to the diazoxon and hydroxydiazinon metabolites and imidacloprid to the 5-hydroxy and olefin metabolites, consistent with a proposed role of CYP6G enzymes in metabolism of phosphorothioate and neonicotinoid insecticides in other species. |
| |
| Keywords: | Cytochrome P450 CYP6G3 Australian sheep blowfly Insecticide resistance Diazinon Imidacloprid 7EC" },{" #name" :" keyword" ," $" :{" id" :" kwrd0050" }," $$" :[{" #name" :" text" ," _" :" 7-ethoxycoumarin BR" },{" #name" :" keyword" ," $" :{" id" :" kwrd0060" }," $$" :[{" #name" :" text" ," _" :" 7-benzyloxyresorufin CuOOH" },{" #name" :" keyword" ," $" :{" id" :" kwrd0070" }," $$" :[{" #name" :" text" ," _" :" cumene hydroperoxide δ-ALA" },{" #name" :" keyword" ," $" :{" id" :" kwrd0080" }," $$" :[{" #name" :" text" ," _" :" delta-aminolevulinic acid DDT" },{" #name" :" keyword" ," $" :{" id" :" kwrd0090" }," $$" :[{" #name" :" text" ," _" :" dichlorodiphenyltrichloroethane DTT" },{" #name" :" keyword" ," $" :{" id" :" kwrd0100" }," $$" :[{" #name" :" text" ," _" :" dithiothreitol EDTA" },{" #name" :" keyword" ," $" :{" id" :" kwrd0110" }," $$" :[{" #name" :" text" ," _" :" ethylenediaminetetraacetic acid fCPR" },{" #name" :" keyword" ," $" :{" id" :" kwrd0120" }," $$" :[{" #name" :" text" ," $$" :[{" #name" :" __text__" ," _" :" cytochrome P450 reductase from " },{" #name" :" italic" ," _" :" Musca domestica IPTG" },{" #name" :" keyword" ," $" :{" id" :" kwrd0140" }," $$" :[{" #name" :" text" ," _" :" isopropyl β-D-1-thiogalactopyranoside IMP" },{" #name" :" keyword" ," $" :{" id" :" kwrd0150" }," $$" :[{" #name" :" text" ," _" :" 2-isopropyl-6-methyl-4-pyrimidinol LC-MS/MS" },{" #name" :" keyword" ," $" :{" id" :" kwrd0160" }," $$" :[{" #name" :" text" ," _" :" liquid chromatography mass spectrometry/mass spectrometry luciferin-BE" },{" #name" :" keyword" ," $" :{" id" :" kwrd0170" }," $$" :[{" #name" :" text" ," _" :" luciferin 6′-benzyl ether luciferin-CEE" },{" #name" :" keyword" ," $" :{" id" :" kwrd0180" }," $$" :[{" #name" :" text" ," _" :" luciferin 6′-chloroethyl ether luciferin-H" },{" #name" :" keyword" ," $" :{" id" :" kwrd0190" }," $$" :[{" #name" :" text" ," _" :" 6′-deoxyluciferin luciferin-H-EGE" },{" #name" :" keyword" ," $" :{" id" :" kwrd0200" }," $$" :[{" #name" :" text" ," _" :" 6′-deoxyluciferin 4-ethylene glycol ester luciferin-ME" },{" #name" :" keyword" ," $" :{" id" :" kwrd0210" }," $$" :[{" #name" :" text" ," _" :" luciferin 6′-methyl ether luciferin-ME-EGE" },{" #name" :" keyword" ," $" :{" id" :" kwrd0220" }," $$" :[{" #name" :" text" ," _" :" luciferin 6′-methyl ether 4-ethylene glycol ester luciferin-PFBE" },{" #name" :" keyword" ," $" :{" id" :" kwrd0230" }," $$" :[{" #name" :" text" ," _" :" luciferin 6′-pentafluorobenzyl ether P450" },{" #name" :" keyword" ," $" :{" id" :" kwrd0240" }," $$" :[{" #name" :" text" ," _" :" cytochrome P450 PMSF" },{" #name" :" keyword" ," $" :{" id" :" kwrd0250" }," $$" :[{" #name" :" text" ," _" :" phenylmethylsulfonyl fluoride pNAOD" },{" #name" :" keyword" ," $" :{" id" :" kwrd0260" }," $$" :[{" #name" :" text" ," $$" :[{" #name" :" italic" ," _" :" p-" },{" #name" :" __text__" ," _" :" nitroanisole " },{" #name" :" italic" ," _" :" O-" },{" #name" :" __text__" ," _" :" dealkylation pNP" },{" #name" :" keyword" ," $" :{" id" :" kwrd0270" }," $$" :[{" #name" :" text" ," $$" :[{" #name" :" italic" ," _" :" p-" },{" #name" :" __text__" ," _" :" nitrophenol pNA" },{" #name" :" keyword" ," $" :{" id" :" kwrd0280" }," $$" :[{" #name" :" text" ," $$" :[{" #name" :" italic" ," _" :" p-" },{" #name" :" __text__" ," _" :" nitroanisole |
| 本文献已被 ScienceDirect 等数据库收录! |
|

