Synthesis of 2,2,4-trimethyl-1,2-dihydroquinolinyl substituted 1,2,3-triazole derivatives: Their evaluation as potential PDE 4B inhibitors possessing cytotoxic properties against cancer cells |
| |
| Affiliation: | 1. Department of Chemistry, MNR Degree & PG College, Kukatpally, Hyderabad 500085, India;2. MSN Pharmachem Pvt. Ltd., Plot No. 212/A,B,C,D, APIICL, Phase-II, Pashamylaram, Patancheru (M), Medak District 502 307, Andhra Pradesh, India;3. Centre for Chemical Sciences and Technology, Institute of Science & Technology, Jawaharlal Nehru Technological University Hyderabad, Kukatpally, Hyderabad 500085, India;4. Chemical Biology Laboratory, Medicinal Chemistry and Pharmacology Division, Indian Institute of Chemical Technology, Tarnaka, Hyderabad 500007, India;5. The University of Queensland, School of Pharmacy, Brisbane, QLD 4072, Australia;6. Department of Chemistry, School of Engineering, Anurag Group of Institutions, R.R.District 501301, Andhra Pradesh, India;2. National and Regional Joint Engineering Laboratory for Medicament of Zoonosis Prevention and Control, Guangzhou 510642, China;3. Key Laboratory of Animal Vaccine Development, Ministry of Agriculture, Guangzhou 510642, China;4. Key Laboratory of Zoonosis Prevention and Control of Guangdong, Guangzhou 510642, China;1. University of Zagreb, Faculty of Chemical Engineering and Technology, Department of Organic Chemistry, Marulićev Trg 20, HR–10000 Zagreb, Croatia;2. University of Rijeka, Department of Biotechnology, Radmile Matejčić 2, HR–51000 Rijeka, Croatia;3. University of Rijeka, Centre for High-throughput Technologies, Radmile Matejčić 2, HR–51000 Rijeka, Croatia;4. University of Zagreb, Faculty of Textile Technology, Department of Applied Chemistry, Prilaz Baruna Filipovića 28a, HR–10000 Zagreb, Croatia;5. Computational Organic Chemistry and Biochemistry Group, Ruđer Bošković Institute, Bijenička 54, HR–10000 Zagreb, Croatia |
| |
| Abstract: | 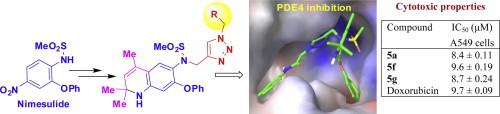
The 2,2,4-trimethyl-1,2-dihydroquinolinyl substituted 1,2,3-triazole derivatives were designed as potential inhibitors of PDE4B. These compounds were synthesized via a multi-step sequence consisting of copper-catalyzed azide-alkyne cycloaddition (CuAAC) as a key step in aqueous media. The required alkynes were prepared from nimesulide via N-propargylation and then nitro group reduction followed by a CAN mediated modified Skraup reaction of the resulting amine. All the synthesized compounds showed PDE4B inhibitory properties in vitro at 30 μM with two compounds showing >50% inhibition that were supported by the in silico docking results of these compounds at the active site of PDE4B. Three of these PDE4 inhibitors showed promising cytotoxic properties against A549 human lung cancer cells in vitro with IC50 ∼8–9 μM. |
| |
| Keywords: | Nimesulide 1,2,3-Triazole Cycloaddition PDE4B Cytotoxic activities |
| 本文献已被 ScienceDirect 等数据库收录! |
|

