Tandem histone folds in the structure of the N-terminal segment of the ras activator Son of Sevenless |
| |
| Authors: | Sondermann Holger Soisson Stephen M Bar-Sagi Dafna Kuriyan John |
| |
| Affiliation: | Howard Hughes Medical Institute, Department of Molecular and Cell Biology, University of California-Berkeley, Berkeley, CA 94720, USA. |
| |
| Abstract: | 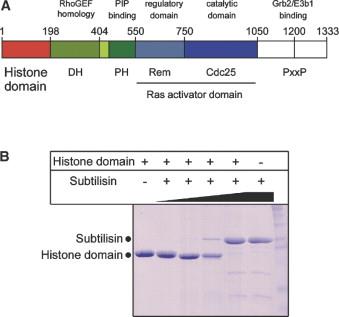
The Ras activator Son of Sevenless (Sos) contains a Cdc25 homology domain, responsible for nucleotide exchange, as well as Dbl/Pleckstrin homology (DH/PH) domains. We have determined the crystal structure of the N-terminal segment of human Sos1 (residues 1-191) and show that it contains two tandem histone folds. While the N-terminal domain is monomeric in solution, its structure is surprisingly similar to that of histone dimers, with both subunits of the histone "dimer" being part of the same peptide chain. One histone fold corresponds to the region of Sos that is clearly similar in sequence to histones (residues 91-191), whereas the other is formed by residues in Sos (1-90) that are unrelated in sequence to histones. Residues that form a contiguous patch on the surface of the histone domain of Sos are conserved from C. elegans to humans, suggesting a potential role for this domain in protein-protein interactions. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect PubMed 等数据库收录! |
|

